Mobile Patient-centred System to Improve Drug Trials and Care of Older-adults with Rheumatic Diseases
Description:
56% of adults have a rheumatic condition and figures increase greatly with age. The high prevalence of rheumatic conditions in older adults is likely to further distance follow up appointments, and thus impact patient care. Moreover, a six-month distance is already likely to miss flares and have low sensitivity, low sampling frequency, high variability, and an inadequate representation of patient experiences, both in clinical care and trials. Electronic patient reported outcomes measures (ePROMS) have been developed to support clinicians with richer outcomes from the patient perspective. However, existing ePROMS are not cost-effective, since they are: i) restricted to self-reported data, leaving behind digital endpoints; ii) difficult to use by patients, which affects mostly older adults with less ICT experience; and iii) targets specific care focuses (e.g. clinical care OR drug trials) disregarding the ecosystem nature of care; iv) are not focused on rheumatic diseases.
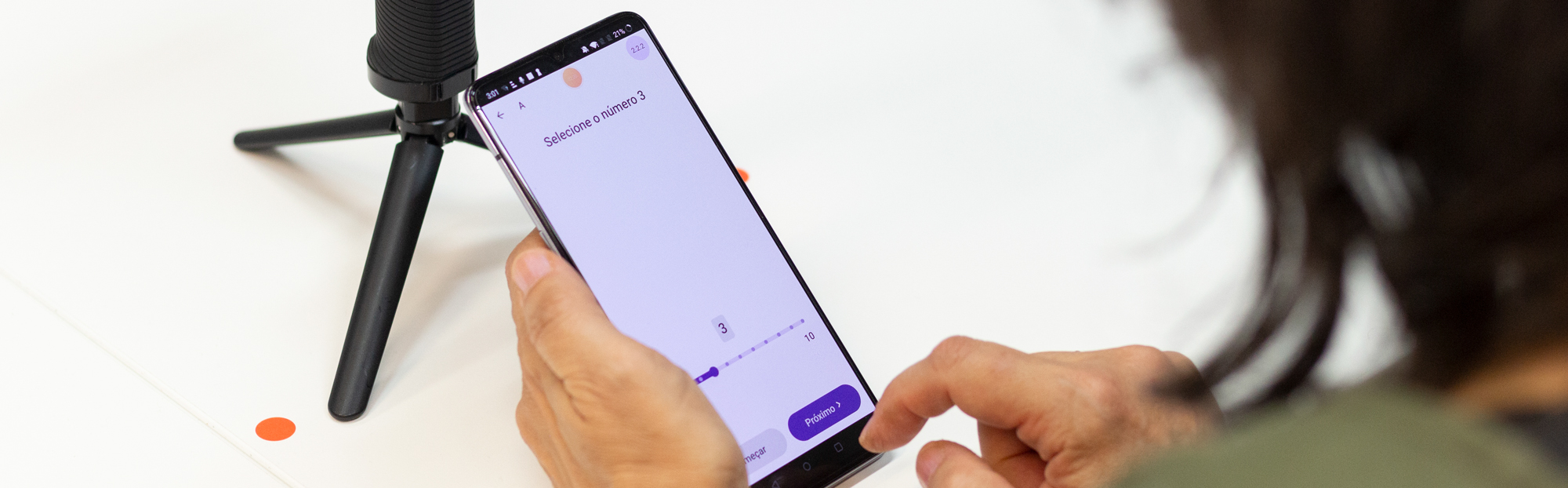
COTIDIANA is developing a cost-effective mobile solution to efficiently acquire PROMs and sensor data to support clinical care and drug trials of older adults, collecting patient data in a more holistic and efficient approach. Patients will use the smartphone in ambulatory conditions to report experiences, symptoms/signs, and quality of life. Drawing on the smartphone’s built-in sensors and logs, we will be able to objectively track digital endpoints focused on hand dexterity, gait and physical activity, and sociability patterns.
The project started with ethnographic and participatory research with with patients, informal carers, and clinicians, as well as with a broad group of stakeholders which include clinical researchers, people from Pharma, or Clinical Research Organizations. Laboratory data collections with patients will also be performed, for training passive sensing algorithms and crafting digital endpoints. In parallel, the project will develop a mobile app for patients and a web app for clinicians and clinical researchers. The developed solution will be assessed in three field trials, one of which focused on technological feasibility and the two others focused on clinical care and clinical research or drug trials.
If successful, the COTIDIANA solution will be commercialized by two companies from the consortium, providing hospitals, clinics, and Pharma, with a solution that provides rich data about the current health state of their patients with rheumatic conditions.
Partners:
Associação Fraunhofer Portugal Research (leader)
Mag. Andreas Raffeiner
Medizinische Universität Wien
Pryv AS
Universidade NOVA de Lisboa
Website:
Co-funded by:

For any additional information, please contact us using the inquiries form.
 Fraunhofer Center for Assistive Information and Communication Solutions – AICOS
Fraunhofer Center for Assistive Information and Communication Solutions – AICOS